ANSWER
The final pressure of the sample is 3.5 atm
EXPLANATIONS
Given that;
The initial volume of the sample is 23.0mL
The initial pressure of the sample is 1.45 atm
The final volume of the sample is 9.5mL
To find the final pressure of the sample, follow the steps below
In the given data, the temperature of the sample is fixed. Hence, we can apply Boyle's law to find the final volume
Step 1; state Boyle's law
Boyle's law states that the volume of a given mass is inversely proportional to its pressure at constant temperature.
Mathematically, the law can be expressed below
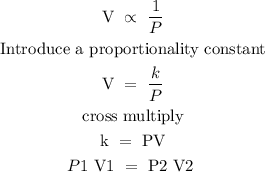
Step 2; Substitute the given data into the formula in step 1

Hence, the final pressure of the sample is 3.5 atm