Option b:
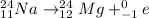
A nuclear equation is said to be balanced if the sum of the mass number A and sum of the atomic number Z is equal on both sides of the reaction arrow.
In nuclear reaction, atoms are represented as follows:
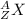
Here, A stands for mass number, Z stands for atomic number and X represents the atomic symbol.
a. The nuclear reaction is as follows:
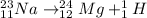
Here, sum of mass number and atomic number on left side of the reaction arrow is 23 and 11 respectively.
On right hand side sum of mass number is 24+1=25 and that of atomic number is 12+1=13. Since, both mass number and atomic number are not same, it is not a balanced nuclear reaction.
b. The nuclear reaction is as follows:
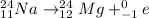
Here, sum of mass number and atomic number on left side of the reaction arrow is 24 and 11 respectively.
On right hand side sum of mass number is 24+0=24 and that of atomic number is 12-1=11. Since, both mass number and atomic number are same, it is a balanced nuclear reaction.
c. The nuclear reaction is as follows:
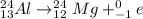
Here, sum of mass number and atomic number on left side of the reaction arrow is 24 and 13 respectively.
On right hand side sum of mass number is 24+0=24 and that of atomic number is 12-1=11. Since, atomic number is not same, it is not a balanced nuclear reaction.
d. The nuclear reaction is as follows:
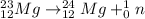
Here, sum of mass number and atomic number on left side of the reaction arrow is 23 and 12 respectively.
On right hand side sum of mass number is 24+1=25 and that of atomic number is 12+0=12. Since, atomic number is not same, it is not a balanced nuclear reaction.