1) Write the chemical equation
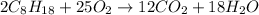
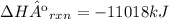
2) Convert grams of octane to moles of octane
The molar mass of octane is 114.33 g/mol
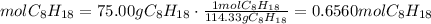
3) Calculate the heat of the reaction
Use the heat of reaction of the balanced reaction to make a conversion factor.
2 C8H18 = -11018kJ
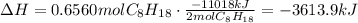
The heat of reaction for 75.00 g of octane is -3614kJ.
.