The question requires us to calculate the amount of energy absorbed by the reaction, considering the molar enthalpy for oxygen gas (O2) and that 80.6 g of oxygen were reacting.
The following information was provided by the question:
Balanced chemical reaction:
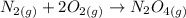
Molar enthalpy of reaction for O2: +499.0 kJ/mol
Mass of O2 reacting: 80.6 g
To solve this problem, we need to calculate the amount of moles that corresponds to the mass of O2 given and then use this value and the molar enthalpy provided to calculate how much heat would be absorbed by the reaction.
First, we need the molar mass of O2. Knowing that the atomic mass of O is 15.99 u:
molar mass (O2) = (2 * 15.99) = 31.98 g/mol
Now, we use the molar mass to calculate the number of moles in 80.6 g of O2:
31.98 g O2 ------------------------ 1 mol O2
80.6 g O2 ------------------------- x
Solving for x, we'll have:
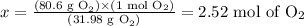
There are 2.52 moles of O2 reacting and next we need to calculate the amount of heat absorbed considering this.
The molar enthalpy of O2 tells us how much heat is abosrbed when 1 mol of O2 reacts. Thus, we can use it to calculate the amount of heat absorbed when 2.52 moles of O2 react:
1 mol O2 ----------------------- 499.0 kJ
2.52 mol O2 ----------------- y
Solving for y, we'll have:
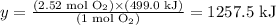
Therefore, 1257.5 kJ of energy are absorbed when 80.6 g of O2 are reacting.
We can fill in the boxes to answer the question as it follows:
Answer: 1257.5
Units: kJ