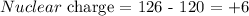Answer:
Step-by-step explanation:
Here, we want to calculate the effective neutral charge
From the question, there are 126 electrons
Since there are 6 valence electrons, the number of electrons residing outside the valence electrons is 120
The effective nuclear charge is the difference between the number of protons and the number of electrons outside the valence shell
Mathematically, we have it that: