Answer: Option (B) is the correct answer.
Step-by-step explanation:
According to law of conservation of mass, mass can neither be created nor it can be destroyed. It can only be transformed from one form to another.
So, it means mass of reactants must be equal to the mass of products in order to follow law of conservation of mass by a chemical reaction.
(a) When 24 g of Mg burn in 32 g
 to produce 56 g of MgO. So, mass of reactants is (24 g + 32 g) = 56 g. Mass of products is 56 g. Hence, it is following law of conservation of mass.
to produce 56 g of MgO. So, mass of reactants is (24 g + 32 g) = 56 g. Mass of products is 56 g. Hence, it is following law of conservation of mass.
(b) 18 g of Mg burn in 24 g
 to produce 24 g of MgO. So, mass of reactants is (18 g + 24 g) = 42 g. Mass of products is 24 g. Hence, it is not following law of conservation of mass.
to produce 24 g of MgO. So, mass of reactants is (18 g + 24 g) = 42 g. Mass of products is 24 g. Hence, it is not following law of conservation of mass.
(c) 2 atoms of Mg react with 1 molecule of
 to produce 2 units of MgO. So, mass of reactants is
to produce 2 units of MgO. So, mass of reactants is
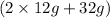 = 56 g. Mass of products is
= 56 g. Mass of products is
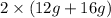 = 56 g. Hence, it is following law of conservation of mass.
= 56 g. Hence, it is following law of conservation of mass.
(d) 1 atom of Mg reacts with 1 atom of O to produce a unit of MgO that contains 2 atoms. So, mass of reactants is
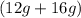 = 28 g. Mass of products is (12 g + 16 g) = 28 g. Hence, it is following law of conservation of mass.
= 28 g. Mass of products is (12 g + 16 g) = 28 g. Hence, it is following law of conservation of mass.
Thus, we can conclude that the equation which does not follow law of conservation of mass is that 18 g of Mg burn in 24 g
 to produce 24 g of MgO.
to produce 24 g of MgO.