Brief script to solve stoichiometry questions
1. Discover the relation in moles between the reactants and products. This is given by the bigger numbers before the formula of the substances.
2. Convert this relation in moles to a relation in grams by multiplying each number of moles by the respective molar masses of each substance.
3. Set a proportion to discover the amount needed of reactants and/or products
Step 1 - Discovering the relation in moles
The given reaction is:
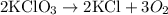
Note there are bigger numbers before the formula of the substances. Respectively: 2, 2 and 3. They indicate a quantity in moles. For this reaction, therefore, we can say that:
2 moles of KClO3 produce 2 moles of KCl and 3 moles of O2
Step 2 - Converting this to a relation in grams
Now let's multiply the number of moles of each substance by their respective molar masses (122 g/mol for KClO3; 32 g/mol for O2):
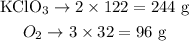
We can see thus that 244 g of KClO3 produce 96 g of O2. This is our recipe for this reaction.
Step 3 - Discovering how many moles of O2 would be produced
Now that we have found a "recipe" for the reaction, we just use its proportion to solve the exercise:
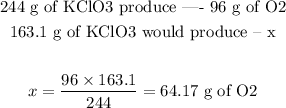
Since the molar mass of O2 is 32 g/mol, 64.17 g is approximately 2 moles of O2. Therefore, 2 moles of O2 could be produced.