Answer:
337.272grams
Explanations:
The balanced chemical equation between lead(IV)oxide and hydrochloric acid is given as;
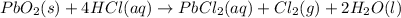
From the reaction, we can see that 1 mole of PbO2 produces 1 mole of chlorine gas (Cl2).
Get the number of moles of Chlorine gas;
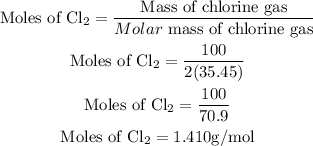
Since the stoichiometric ratio of PbO2 and the chlorine gas is 1:1, hence the number of moles of lead(IV) oxide will be 1.410g/mol
Get the molar mass of lead(IV)oxide;
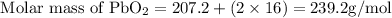
Next is to get the required mass of lead (IV) oxide that is needed to produce 100 grams of chlorine gas as shown;
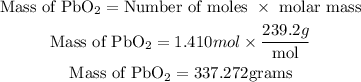
Therefore the mass of lead (IV) oxide needed to produce 100 grams of chlorine gas is 337.272grams