Answer: option A Due to its polarity and hydrogen bonding water can absorb heat without a significant temperature change.
A water molecule
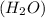 is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances.
is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances.