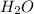Answer: The coefficient '2' must be added in front of
Step-by-step explanation:
Every balanced chemical equation follows law of conservation of mass.
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on the product side.
The given chemical equation follows:
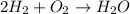
On the reactant side:
Number of hydrogen atoms = 4
Number of oxygen atoms = 2
On the product side:
Number of hydrogen atoms = 2
Number of oxygen atoms = 1
We need to add a coefficient '2' in front of
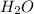 to make the atoms balanced on both sides of the reaction.
to make the atoms balanced on both sides of the reaction.
Hence, the coefficient '2' must be added in front of