Answer:
56 L of

Step-by-step explanation:
To solve this question the first step will be to verify that the chemical equation is balanced. As this equation is balanced we can proceed with the following steps:
1) To get the liter of oxygen gas to be collected we can employ the formula: pV=nRT where:
a) p= Pressure (1 atm at STP (Standard Preassure and Temperature)
b)V= volume (in litters)
c) n= number of moles
d) R= The gas constant that for STP conditions is 0.082 L atm mol
 K
K

e) T=temperature (273.5 K for STP conditions)
From this formula we can get the number of litters by: V=
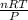
To calculate the number of moles we have that 2 moles of
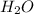 equal on mole of
equal on mole of
 . From the question we have that 90 grams o water are used for this reaction. To pass from grams to moles we can use the molecular weight, which for water is 18
. From the question we have that 90 grams o water are used for this reaction. To pass from grams to moles we can use the molecular weight, which for water is 18
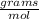 . Therefore,
. Therefore,
The number of moles of water= 90grams x(
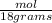 = 5 moles of
= 5 moles of
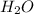 . We know that 2 moles of water equal one mole of oxygen gas, for this, 5 moles of water equal 2.5 moles of oxygen gas.
. We know that 2 moles of water equal one mole of oxygen gas, for this, 5 moles of water equal 2.5 moles of oxygen gas.
With the number of moles of oxygen gas calculated, we can substitute all the elements in the formula of the ideal gas to obtain the number of liters:
V =
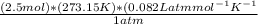 ; therefore,
; therefore,
V= 56.0 L of
