Answer:
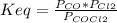
Step-by-step explanation:
A reaction is said to be at equilibrium when the rate of consumption of reactants equals the rate of formation of products. This state can be depicted in terms of the equilibrium constant (Keq) which is essentially the ratio of concentration (or pressure for gases) of the products to that of the reactants each of which is raised to an appropriate coefficient defined by the balanced chemical equation.
![Keq = ([Products])/([Reactants])](https://img.qammunity.org/2018/formulas/chemistry/high-school/9mgi0r8yfbgig3pfhs1n0qxrkiyeq0agzb.png)
The given reaction is:
cocl2(g) ↔ co(g) + cl2(g)
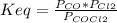
Here the Keq is given in terms of the partial pressure of the gases