Answer:- (1) 3.00 moles of KOH in 1.0 kg of water (2) 0.40 mole of
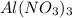 in 1.0 kg of water.
in 1.0 kg of water.
Explanations:- Elevation in boiling point is directly proportional to the molality of the solution. The equation used for this is written as:

where,
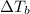 is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and
is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and
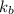 is the molal elevation constant for the solvent used.
is the molal elevation constant for the solvent used.
Van't hoff factor is the theoretical number of ions an ionic compound give when it breaks. For example NaCl breaks to give
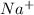 and
and
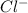 . So, the value of i for NaCl is 2.
. So, the value of i for NaCl is 2.
In the first pair we have LiOH and KOH. LiOH gives lithium ion and hydroxide ion, so the value of i for this is 2. Similarly, KOH gives potassium ion and hydroxide ion and the value of i for this is also 2.
So, for this pair the deciding parameter is the molality. Molality of KOH is higher than LiOH and so the boiling point will be higher for KOH.
For second pair, the molality is same for both the solutions so the deciding parameter here is the value of van't hoff factor. CsCl breaks to give cesium ion and chloride ion and so the value of i for this is 2.
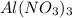 breaks to give one
breaks to give one
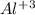 ion and three
ion and three
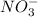 ions. So, the value of i for this is 4.
ions. So, the value of i for this is 4.
Since the value of i is higher for aluminium nitrate, it's boiling point will be higher.