Answer:
The balanced chemical equation is ;
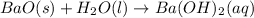
Explanation:
When barium hydroxide react with water it gives an alkaline solution of barium hydroxide.
The balanced chemical equation is given as:
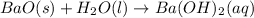
according to stoichiometry,when 1 mole of barium oxide reacts with 1 mole of water it gives 1 mole of barium hydroxide.