Answer:-
1. one mole of any gas at stp will occupy 22.4 l : avogadro's hypothesis
2. the sum of the protons and neutrons in an atom: Mass number
3. 6.02 x 1023; the number of atoms, molecules, or particles in a mole : avogadro's number
4. atoms of the same element that have different numbers of neutrons : isotopes
5. the relative mass of an atom as compared to the carbon-12 atom: molar mass
6. the amount of a substance that contains the same number of atoms, molecules, or particles as 12 g of carbon-12: mole
7. the mass of an element or molecule in grams per mole (g/mol): atomic mass
Explanation:-
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP and contains avogadro's number
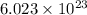 of particles.
of particles.
Avogadro's number of substance represents 1 mole and thus weigh same as molecular mass called as molar mass. Molar mass is expressed in g/mol.
Protons are nucleus are located in the center of an atom where while mass of an atom is concentrated.
Mass number = Number of protons + Number of neutrons
Mass number is a whole number.
Isotopes are the atoms of same element with same atomic number but different mass number. Thus they have same protons but different number of neutrons.
Atomic mass is calculated by:
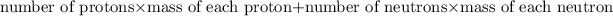 . It is a integer.
. It is a integer.