Answer: Option (b) is the correct answer.
Step-by-step explanation:
When there occurs sharing of electrons between two chemically combining atoms then it forms a covalent bond.
As, atomic number of hydrogen is 1 and its electronic configuration is
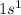 .
.
Hence, in order to become stable in nature it will reaction share its valence electron with another atom.
As atomic number of chlorine is 17 and its electronic distribution is 2, 8, 7. So, in order to attain stability it will readily react a hydrogen atom who also wants to become stable.
As a result, HCl compound will be formed.
Whereas Na, B, and Li being metals will donate their valence electrons and form an ionic bond.
Thus, we can conclude that with Cl atom would a hydrogen atom share its valence electrons.