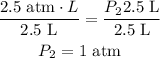Answer
1 atm
Step-by-step explanation
Given:
V₁ = 5 L P₁ = 0.5 atm V₂ = 2.5 L P₂ = ?
According to Boyle's law, the pressure (P) of a given quantity of gas varies inversely with its volume (V) at constant temperature; i.e., in equation form,
P₁V₁ = P₂V₂
So substitute the given values into the equation to get P₂
0.5 atm x 5 L = P₂ x 2.5 L
2.5 atm.L = P₂ 2.5 L
Divide both sides by 2.5 L