When Temperature increases the velocity of the molecules increases, since the kinetic energy is given by:
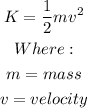
The velocity is directly proportional to the kinetic energy, therefore, the kinetic energy increases too.
Answer:
b. The average kinetic energy of water molecules in picture 1 is greater than those in picture 2.