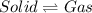Answer: Melting, vaporization and sublimation.
Step-by-step explanation: Melting is a process in which solid phase changes to liquid phase by absorbing heat and thus the particle atoms overcome the attractive forces between solids and move away from each other in liquids. The reverse process is freezing in which particles come close to each other.

Vaporization is a process in which liquid phase changes to gaseous phase by absorbing heat and thus the particle atoms overcome the attractive forces between liquids and move away from each other in gaseous phase.The reverse process is condensation in which particles come close to each other.
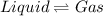
Sublimation is is a process in which solid phase directly changes to gaseous phase by absorbing heat and thus the particle atoms overcome the attractive forces between solids and move away from each other in gaseous phase.The reverse process is deposition in which particles come close to each other.