The question is incomplete, here is a complete question.
The given chemical reaction is:
Reaction 1 :
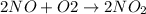 ΔH = +109 kJ/mol
ΔH = +109 kJ/mol
What is the enthalpy for reaction 1 reversed?
Answer : The enthalpy of the reaction will be, -109 kJ/mol
Explanation :
The given chemical reaction is:
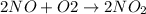
The enthalpy of given chemical reaction is, +109 kJ/mol.
As we know that, when we are reversing the reaction then the sign of the enthalpy change of the reaction will also changed. That means, positive becomes negative and negative becomes positive.
That means,
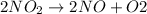 ΔH = -109 kJ/mol
ΔH = -109 kJ/mol
Hence, the enthalpy of the reaction will be, -109 kJ/mol