Answer:
4.62 g of Ag (silver).
Step-by-step explanation:
What is given?
Mass of Cu (copper) = 734 g.
Mass of AgNO3 (silver nitrate) = 7.27 g.
Molar mass of Cu = 63.5 g/mol.
Molar mass of AgNO3 = 170 g/mol.
Molar mass of Ag = 108 g/mol.
Step-by-step solution:
First, let's state the chemical equation with copper (Cu) and silver nitrate (AgNO3) reacting:
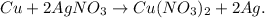
Now, let's find the number of moles of each reactant using their molar mass:
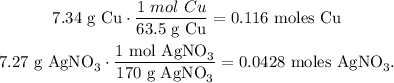
Now, let's see how many moles of Ag are being produced based on the number of moles that we found in each reactant.TBy doing this calculation, we will find the limiting reactant.
You can see in the chemical equation that 1 mol of Cu reacted, produces 2 moles of Ag, so the moles of Ag produced are:
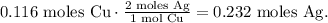
And you can see that moles of Ag NOa reacte, produces2 moles of Ag too. This means that the mola r ratio betwee tehem is 22, more simply sis 1:1. This is telling u that 0.0428 moles of AgNO3 will produce 0.00428 moles of Ag too.
In this case, as you can see the limiting reactant is AgNO3because this is tbeing consumed first in the reaction, so the final step is to find the mass using its molar mass. The conversion from 0.0428 moles of Ag to mass in grams is:
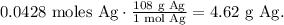
The answer is that we're producing 4.62 g of Ag (silver).