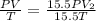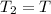Answer: The temperature will not change and will remain T.
Step-by-step explanation: Accoding to ideal gas law:
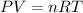
where,
P = pressure of the gas
V = volume of the gas
T = temperature of the gas
n = number of moles of gas
R = Gas constant = 0.0821 Latm/moleK
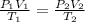
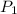 = initial pressure
= initial pressure
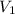 = initial volume
= initial volume
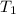 = initial temperature
= initial temperature
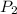 = final pressure
= final pressure
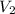 = final volume
= final volume
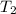 = final temperature
= final temperature
Putting in the values: