Answer:
Q = 1086.75 [J]
Step-by-step explanation:
We must use the following equation of thermal energy, which relates the specific heat to the mass and to the change in temperature.
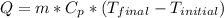
Where:
m = mass = 15.75 [g]
Cp = specific heat = 0.46 [J/g*C]
Tfinal = final temperature = 175 [°C]
Tinitial = initial temperature = 25 [°C]
Now replacing:
![Q=15.75*0.46*(175-25)\\\\Q= 1086.75[J]\\](https://img.qammunity.org/2022/formulas/physics/high-school/xf9imdqt8ctf769csuujmephnzp8iu8fsv.png)