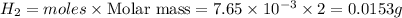Answer:- 0.0153 grams
Explanation:- According to Dalton's law, the total pressure is the sum of individual pressures.
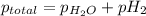
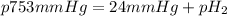
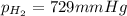
According to the ideal gas equation:'
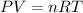
Pressure of the gas = 729 mmHg = 0.96 atm (1mmHg=0.0013atm)
Volume of the gas = 195 mL = 0.195 L
Temperature of the gas = 25°C =(25+273)= 295K (0°C = 273 K)
R= gas constant = 0.0821Latm\Kmol

Mass of