Being given the hydronium (or simply the hydrogen) ion concentration (molarity), one of the easiest properties of the solution to determine is its pH. The pH of a solution indicates the acidity (or basicity) of a given solution. The typical pH range of solutions is from 1 to 14, with 7 being neutral, 1 to 6 being acidic, and 8 to 14 being basic.
To determine the pH of a solution, the negative log (-log) of the hydronium/hydrogen ion concentration (molarity) of the solution is calculated. Thus (assuming that the given hydrogen ion concentration is in molarity (mol/L)),
pH = -log(
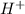
) or -log(
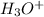
pH = -log (
7.94 × 10−6 mol/L)
pH = 5.13
Because the pH is less than 7 (within the range 1 to 6), then the black coffee solution is acidic.