We will assume that the gas behaves like an ideal gas, so we can apply Charles's law in this case, which relates volume to the temperature at constant pressure. Charles's law tells us that:
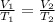
where,
V1 is the initial volume of the gas, 40 mL=0.04L
T1 is the initial volume of the gas
T2 is the final temperature of the gas, 2xT1
V2 is the final volume of the gas
If we clear V2 and replace T2=2xT1 we will have:
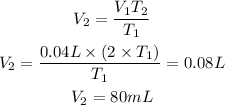
The volume would be 80mL, answer is the third option