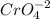
Because the ratio of the chromium to oxygen should be 1 is to 4, the first formula that comes to mind is
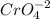
, with 1 chromium atom and 4 oxygen atoms.
The most common oxidation states of chromium are +6, +3, and +2. Less common are the oxidation states +5, +4, and +1 states, which are present in a few stable compounds.
The oxide ion has a charge of -2. With the chromate formula being
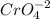
, the chromium oxidation state (x) is calculated to be
x + 4*(-2) = -2
x = -2+8
x = +6
The chromium oxidation state is +6, which is a known common oxidation state of chromium.
Testing other 1:4 ratios of Cr and O by multiplying the ratio with different factors would give,
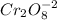
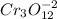
and so on.
Determining the oxidation state (x) of chromium for the two possible chromate ion formulas,
1.
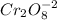
2x + 8*(-2) = -2
2x = -2+16
2x = +14
x = +7
2.
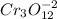
3x + 12*(-2) = -2
3x = -2+24
3x = +22
x = +7.3
As observed, using chromate ion formulas in which the 1:4 ratio is multiplied by factors greater than 1, the calculated oxidation states of chromium are not considered to be possible. Therefore, the only possible formula for the chromate ion with a chromium to oxygen ratio of 1:4, and an ionic charge of -2 is
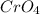
.
.