Answer:
2 is the van’t Hoff factor for NaCl if it completely dissociates in water.
Explanation:
Formula used :
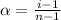
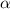 = Degree of dissocation.
= Degree of dissocation.
n = number of ions produced by dissociation of ionic compound
i = van't Hoff factor
We have:
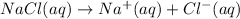
n = 2.
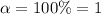
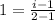
i = 2
2 is the van’t Hoff factor for NaCl if it completely dissociates in water.