To solve this question, the first step is to convert both masses to moles of each reactant (use the molecular mass of each compound):
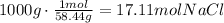
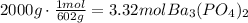
Now, we have to use the mole ratio of NaCl to Ba3(PO4)2 to determine how much of one of the reactants is needed to react with the given amount of the other reactant:
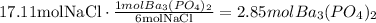
It means that 17.11 mol of NaCl react with 2.85 mol of Ba3(PO4)2, it means that the limiting reactant is NaCl (Ba3(PO4)2 is in excess).
If we have 3.32 moles of the excess reactant and we only need 2.85, we have 0.47 moles left. This converted to grams (using the molecular mass of barium phosphate) is:
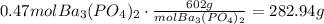
There are 282.94 grams of excess reactant left.