Answer
The moles of sodium needed to produce 2.83 grams of sodium oxide = 0.0914 moles.
Step-by-step explanation
Given:
Mass of sodium oxide produced = 2.83 grams
What to find:
The moles of sodium needed to produce 2.83 grams of sodium oxide.
Step-by-step solution:
Step 1: Step 2: Write a balance equation for the reaction.
4Na + O₂ -------> 2Na₂O
Step 2: Convert 2.83 grams sodium oxide to mole using the mole formula.
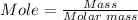
The molar mass of Na2O = 61.9789 g/mol
So, putting mass = 2.83 g and molar mass = 61.9798 g/mol, we have;
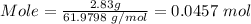
Step 3: Calculate the mole of sodium needed using the balance equation and the mole of sodium oxide produced.
From the balanced equation;
4 moles Na produces 2 moles Na₂O
So, the moles of Na needed to produce 0.0457 moles Na₂O will be
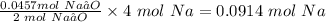
The moles of sodium needed to produce 2.83 grams of sodium oxide = 0.0914 moles