The question gives us the reaction to produce sodium sulfide (Na2S) from sodium sulfate (Na2SO4) and carbon (C) and provides the amount of reactants used (15.0 g of Na2SO4 and 7.50 g of C), asking the limiting reactant, the excess reactant and the amount of Na2S produced.
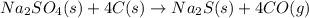
I) The first step for this type of question is checking if the given equation is balanced. For this case, we don't need to adjust the coefficients as the equation is already balanced.
II) Next, we need to calculate the molar mass of the compounds we'll be using. To calculate the molar mass, I'll be using the following atomic masses and considering the number of each atom in the molecules:
Na: 22.99 u
S: 32.07 u
O: 15.99 u
C: 12.01 u
Now, we can calculate the molar mass of Na2SO4, C and Na2S:
Na2SO4: molar mass = (2 * 22.99) + (1 * 32.07) + (4 * 15.99) = 142.01 g/mol
C: molar mass = (1 * 12.01) = 12.01 g/mol
Na2S: molar mass = (2 * 22.99) + (1 * 32.07) = 78.05 g/mol
iii) The third step is to convert the masses given for Na2SO4 and C into the correspondent number of moles using the molar mass of these compounds:
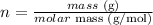
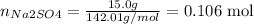
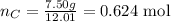
IV) On the forth step, we must define the limiting reactant for this reaction considering the amounts used of each one and the stoichiometric coefficients:
1 mol Na2SO4 reacts with 1 mol C
0.106 mol Na2SO4 reacts with...?
Solving this calculation, we have that we would need 0.106 mol of C to react with 15.0 g of Na2SO4. Since we there are 0.624 mol of C available to react, we can conclude that carbon is the reactant in excess (there is an excess of 0.518 mol) and sodium sulfate is the limiting reactant.
V) At last, we can calculate the amount of Na2S produced from the limiting reactant amount used (0.106 mol of Na2SO4) and the stoichiometric coefficients:
1 mol Na2SO4 ----------1 mol Na2S
0.106 mol Na2SO4 ---- y
Solving for y, we have that 0.106 mol of Na2S will be produced.
vWe can convert this amount into mass of Na2S using its molar mass:
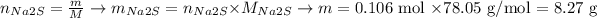
In summary:
Na2SO4 is the limiting reactant and there is an excess of 0.518 mol or 6.22 g of C;
8.27 g of Na2S will be produced from 15.0 g of Na2SO4.