Chemistry => Oxidation-Reduction Reactions => Assigning Oxidation Numbers
The oxidation number of a chemical element is an expression of how oxidized the element can be, that is, how many electrons it gained or lost.
When an element is in its natural state, its oxidation state is zero. A compound also has an overall oxidation state of zero, so the oxidation state of the elements must be such that the sum is zero.
There are some elements that have only one oxidation state, we can start with these elements and calculate the rest.
These elements that acquire a unique oxidation state when they are linked are:
Al : 3+
H: 1+
With this information we can complete all the oxidation states as follows:
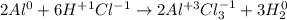
We see that Al and H2 in their natural state have an oxidation state equal to 0. The oxidation state of chlorine was deduced by difference, making the molecule have an overall oxidation state equal to zero.
In this way, the answer will be:
To know the electrons gained or lost, we must see how the oxidation states of the elements change.
Aluminum goes from 0 to +3, that is, it loses 3 electrons. Since there are two aluminum atoms, a total of 6 electrons are lost.
The hydrogen goes from +1 to an oxidation state equal to zero, that is, it gains 1 electron. Since there are 6 hydrogen atoms, a total of 6 electrons are gained.
Therefore, another part of the answer will be:
For the reaction as written, the total number of electrons lost is six and the total number of electrons gained is six