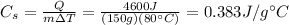Answer:
0.383 J/g°C
Step-by-step explanation:
The heat emitted by the copper ball while cooling down is given by:
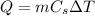
where:
m = 150 g is the mass of the ball
Cs = ? is the specific heat of copper
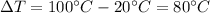 is the change in temperature
is the change in temperature
We know that the heat lost by the copper ball is
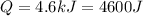
So we can re-arrange the formula to find the specific heat capacity: