Answer
The moles of Mg that reacted = 0.005 moles
The moles of H₂SO₄ that reacted = 0.005 moles.
Justification: The mole ratio from the balanced equation is 1 : 1 and since the moles of H₂SO₄ (0.005 mol is less than 0.020571898 mol of Mg) is the limiting reactant, it determines when the reaction goes into completion.
Step-by-step explanation
Given:
Mass of magnesium that reacts = 0.50 g
The volume of sulfuric acid that reacts = 25 cm³
Molarity of sulfuric acid that reacts = 0.20 moldm⁻³
What to find:
The number of moles of magnesium and sulfuric acid that reacted.
Step-by-step solution:
Step 1: Write the balanced chemical equation for the reaction.
Mg + H₂SO₄ → H₂ + MgSO₄
Step 2: Convert the mass of Mg and molarity of H₂SO₄ to moles.
The mass of Mg can be converted to moles using the mole formula:
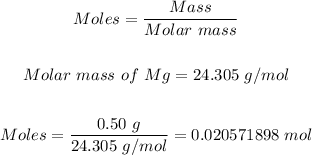
25 cm³ of 0.20 moldm⁻³ of H₂SO₄ can be converted to moles using the formula for molarity:
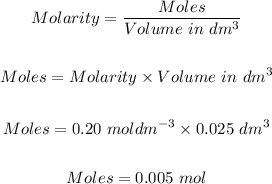
Step 3: Calculate the number of moles of magnesium and sulfuric acid that reacted.
From step 2: moles of Mg = 0.020571898 mol, moles of H₂SO₄ = 0.005 mol
Using the mole ratio of Mg and H₂SO₄ from the balanced equation in step 1, that 1 mol Mg = 1 mol H₂SO₄
Therefore, the moles of Mg that reacted will be 0.005 moles, and the moles of H₂SO₄ that reacted will be 0.005 moles.
Justification: The mole ratio from the balanced equation is 1 : 1 and since the moles of H₂SO₄ (0.005 mol is less than 0.020571898 mol of Mg) is the limiting reactant, it determines when the reaction goes into completion.