Answer : The moles of oxygen in the sample was 0.216 mole.
Explanation : Given,
Moles of C = 0.217 mol
Moles of H = 0.433 mol
Mass of unknown compound = 6.50 g
Molar mass of C = 12 g/mol
Molar mass of H = 1 g/mol
Molar mass of O = 16 g/mol
First we have to calculate the mass of C and H.
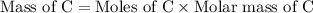
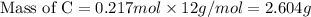

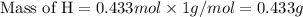
Now we have to calculate the mass of oxygen.
Mass of oxygen = Mass of unknown compound - [Mass of C + Mass of H]
Mass of oxygen = 6.50 - [2.604 + 0.433]
Mass of oxygen = 3.463 g
Now we have to calculate the moles of oxygen.

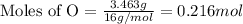
Therefore, the moles of oxygen in the sample was 0.216 mole.