Answer : The molar mass of unknown gas is 99.8 g/mole.
Explanation :
Using ideal gas equation:
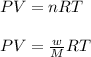
where,
P = pressure of gas = 1.04 atm
V = volume of gas =
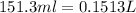
conversion used :
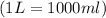
T = temperature of gas =
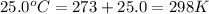
R = gas constant = 0.0821 L.atm/mole.K
w = mass of an unknown gas = 0.642 g
M = molar mass of an unknown gas = ?
Now put all the given values in the ideal gas equation, we get:
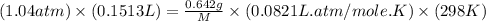
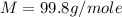
Therefore, the molar mass of unknown gas is 99.8 g/mole.