Answer :
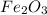 is the product.
is the product.
Explanation :
Balanced chemical reaction : It is defined as the number of atoms of individual elements present on reactant side must be equal to the product side.
The species present on the left side of the right arrow are known as reactant and the species present on the right side of the right arrow are known as product.
When iron react with oxygen to give iron(III) oxide as a product.
The balanced chemical reaction will be,
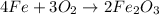
In this reaction,
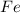 and
and
 are the reactants and
are the reactants and
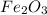 is the product.
is the product.