Answer: 94.9 mL of the concentrated HNO3 solution should be added to water to prepare 500.0 mL of 3.0 M HNO3 solution
Step-by-step explanation:
The question requires us to determine the volume of concentrated nitric acid necessary to prepare a more diluted solution.
The following information was provided by the question:
concentration of stock nitric acid = C1 = 15.8 M
concentration of prepared solution = C2 = 3.0 M
volume of prepared solution = V2 = 500.0 mL
To determine the volume of concentrated nitric acid required, we can apply the following equaiton:
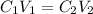
where C1 and V1 are the concentration and volume of the initial solution, and C2 and V2 are the concentration and volume of final solution.
We can rearrange the equation above to calculate the volume of initial (more concentrated) solution:
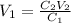
Note that, for the rearranged equation above, if we use the volume V2 in mL, the volume V1 will be obtained in mL - there's no need to convert the volume given, V2 = 500.0 mL, to L, as the units of C2 and C1 will cancel each other.
Applying the values provided by the question, we'll have:
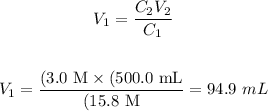
Therefore, 94.9 mL of the concentrated HNO3 solution are required to prepare 500.0 mL of 3.0 M HNO3 solution.