The question requires us to balance the chemical equation through the algebraic method. The question also informs that the value of variable A should be 2.
We can write the chemical equation provided as it follows, already assigning algebraic variables as stoichiometric coefficients to each species:
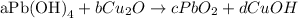
(nothe that the variables assigned are a, b, c and d).
In the algebraic method, we assign variables as coefficients to each species in the reaction in order to obtain mathematical equations that will be solved to obtain the stoichiometric coefficients. We must analyze the amount of atoms of each element on both sides of the chemical equation to obtain the mathematical equations of each element.
For the reaction given, we can take the following mathematical equations:
- For Pb: 1a = 1c
- For O: 4a + 1b = 2c + 1d
- For H: 4a = 1d
- For Cu: 2b = 1d
Next, considering the system of equations obtained, we must find the solution with minimal values for the variables.
Since the question states that a = 2, we can take from the first mathematical equation that:
1a = 1c --> a = c = 2.
From the third equation, we can take that:
4a = 1d --> 4x2 = d --> d = 8
And from the last equation, we can take:
2b = 1d --> 2b = 8 --> b = 4
Therefore, we can assume these values for the variables a, b, c and d: 2, 4, 2 and 8.
Note that we can divide all values by 2 and obtain the results 1, 2, 1 and 4 for the variables a, b, c and d, respectively, which would correspond to the smallest stoichiometric coefficients for the chemical equation.
Now, we can write the values found for the variables as coefficients for the chemical equation and check if it is correctly balanced:
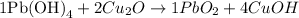
Looking at the chemical equation, we can see that there are:
- 1 atom of Pb on both sides of the equation;
- 4 atoms of Cu on both sides of the equation;
- 4 atoms of H on both sides of the equation;
- 6 atoms of O on both sides of the equation.
Therefore, the reaction is balanced with the coefficients 1, 2, 1 and 4 for Pb(OH)4, Cu2O, PbO2 and CuOH, respectively.