Answer: The correct answer is Nitrogen.
Step-by-step explanation:
Electronic configuration is the representation which helps in determination of the total number of electrons and valence electrons that are present in an element.
For the given options:
Option 1: Carbon having atomic number 6
Electronic configuration of C =
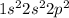
It has 2 electrons in its p-orbital.
Option 2: Oxygen having atomic number 8
Electronic configuration of O =
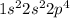
It has 4 electrons in its p-orbital.
Option 3: Nitrogen having atomic number 7
Electronic configuration of N =
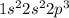
It has 3 electrons in its p-orbital.
Option 4: Beryllium having atomic number 4
Electronic configuration of Be =
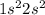
It has 0 electrons in its p-orbital.
From the above information, the correct answer is Nitrogen.