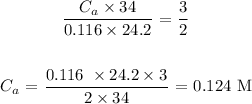Answer:
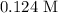
Step-by-step explanation:
Here, we want to get the molarity of sulfuric acid
We start by writing the equation of reaction:
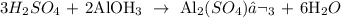
Mathematically, we have it that:
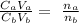
Ca is the molarity of the acid which is unknown
Cb is the molarity of the base which is 0.116 M
Va is the volume of the acid which is 34 mL
Vb is the volume of the base which is 24.2 mL
na is the number of moles of acid in balanced reaction which is 3
nb is the number of moles of base in balanced reaction which is 2
Substituting these values: