Answer:
2.55 g of CO2.
Step-by-step explanation:
What is given?
Volume of HNO3 solution = 350 mL = 0.350 L.
Molarity of HNO3 solution = 0.330 M.
Molar mass of CO2 = 44 g/mol.
Step-by-step solution:
To find how mass of CO2 will be produced based on the volume and molarity of a solution with a excess reactant (Na2CO3), we have to find the number of moles of the substance in the solution, i.e., the number of moles of HNO3. To do this, we use the molarity formula, which is the following:
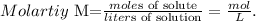
The molarity of the HNO3 solution is 0.330 M, and the volume in liters would be 0.350 L (remember that 1 L equals 1000 mL, so 350 mL = 0.350 L). Let's solve for 'moles of solute' and replace the data that we have, like this:
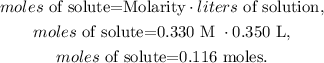
We have 0.116 moles of HNO3 reacting with excess Na2CO3. You can see in the chemical equation that 2 moles of HNO3 reacted produces 1 mol of CO2, so let's see how many moles of CO2 can be produced by 0.116 moles of HNO3:
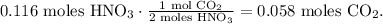
We're producing 0.058 moles of CO2.
The final step is to convert from 0.058 moles of CO2 to grams using the molar mass of CO2, as follows:
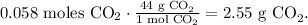
The answer would be that we're producing 2.55 g of CO2.