Answer :16.58 grams of CBr4
Step-by-step explanation
(i) The balanced equation will be as follows:
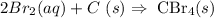
(ii) Determine Moles of Bromine
• Molecular mass Bromine =79,904 g/mol
,
• Mass of Bromine = 8 g
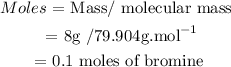
(iii) Determine moles of Carbon tetrabromide :
By stoichiometry, we can determine that :
• 2 moles bromine produces 1 mole CBr4
,
• 0.1 mole bromine will produce X moles CBr4
Therefore , moles of CBr4 = (0.1 mole Br * 1 mole CBr4 )/2 moles Br
= 0.05 moles
(iv) Calculate mass of CBr4
• moles of CBr4 = 0.05moles
• Molecular mass CBr4 =331,63 g/mol
Therefore , Mass of CBr4 is
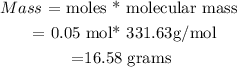
This means that 16.58 grams of CBr4 will be produced from 8 grams of bromine.