The question requires us to complete the sentence regarding the preparation of a more dilute NaOH solution (0.100 M, 50.0 mL) from a more concentrated NaOH solution (1.00 M).
Analyzing the blank spaces that we need to fill in the sentence, we can see that we must provide the volume of the more concentrated solution and the volume of water necessary to prepare the solution.
We can use the following equation to calculate the volume of more concentrated solution required:
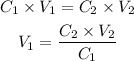
where C1 is the concentration of the initial solution (C1 = 1.00 M), V1 is the volume required of the inital solution (that we'll calculate), C2 is the concentration of the final solution (C2 = 0.100 M) and V2 is the volume of the final solution (V2 = 50.0 mL).
Applying the values given by the question to the equation above, we'll have:
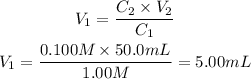
Thus, we would need 5.00 mL of the more concentrated solution.
Since the volume of the final solution is 50.0 mL and it corresponds to the volume of initial solution + volume of water, we can calculate the volume of water necessary as:
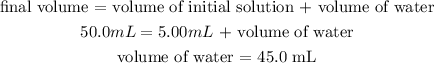
Thus, we would need 45.0 mL of water to prepare the solution.
Therefore, we can complete the sentence given as:
"In order to prepare 50.0 mL of 0.100 M NaOH you will add 5.00 mL of 1.00 M NaOH to 45.0 mL of water"