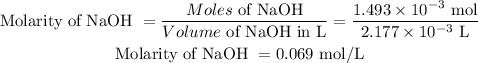Answer
The molarity of NaOH to three decimal places is 0.069 mol/L
Step-by-step explanation
Given:
Volume of NaOH used = 21.77 mL
Reacting mass of KHP = 0.305 g
Molecular Mass of KHP = 204.22 g/mol
What to find:
The molarity of NaOH to three decimal places.
Step-by-step solution:
The first step is to write the balanced stoichiometric chemical equation of the reaction:
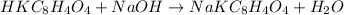
From the balanced chemical equation;
1 mol KHP reacts with 1 mol NaOH
Thus, moles of KHP is:
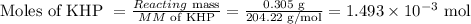
Since, 1 mol KHP reacts with 1 mol NaOH from the balanced equation,
Therefore, 1.493 x 10⁻³ mol KHP will react with:
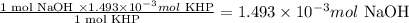
The last step is to calculate the molarity of NaOH:
Conversion factor:
1 mL = 10⁻³ L
21.77 mL = 2.177 x 10⁻³ L
Molarity of NaOH is: