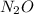Answer:
Step-by-step explanation:
1. First suppose that the mass of the compound is 100g, so:
mass of N + mass of O = 100g
2. Multiply the percentages of N and O by the total mass of the compound:
For the N:
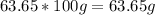 of N
of N
For the O:
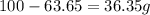 of O
of O
3. Divide the mass of each atom between the molar mass:
For the N:
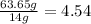
For the O:
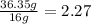
4. Divide each value by the smallest:
For the N:
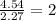
For the O:
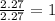
5. Write each atom with its number to obtain the molecular formula: