Answer : The balanced chemical reaction is,
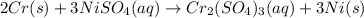
Explanation :
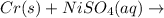
The balanced chemical reaction is,
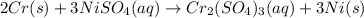
This is a single step displacement reaction. From the reactivity series of the metals, the reactivity of the chromium metal is higher than the reactivity of the nickel metal. Therefore, the chromium metal easily displaces the nickel metal from the solution of nickel(II) sulfate. The reactivity series image attached below.