Answer:
7 protons and 9 neutrons
Step-by-step explanation:
As we know,
Atomic number of an element is the total number of proton in its atom. Thus,
Number of proton
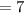
Mass number is equal to the sum of number of proton and number of neutron
Mass number
 Number of Proton
Number of Proton
 number of neutron
number of neutron
Putting the available values in above relation, we get -
 Neutron
Neutron
Neutron
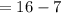
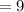
Hence there are 7 protons and 9 neutrons