According to the given reaction, 1 mole of Mg3N2 reacts with 3 moles of H2O.
This, converted to mass, is 101g of Mg3N2 react with 54g of H2O.
From this we can conclude that the limit reagent is Mg3N2.
We have to base our calculation on the mass of Mg3N2 given.
During the reaction, 3 moles of MgO are produced from 101g of Mg3N2. This, converted to mass, is 120.9g of MgO.
a. Use this ratio to find how many grams of MgO are produced from 3.82 grams of Mg3N2:
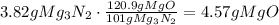
b. To find the percent yield, divide the mass of MgO formed by the theoretical mass of MgO and multiply times 100:
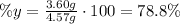
The percent yield is 78.8%.