Answer: Option (d) is the correct answer.
Step-by-step explanation:
A molecule of water
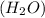 has two hydrogen atoms and one oxygen atom. Since oxygen is more electronegative than hydrogen so, it will attract the electrons from hydrogen atom towards itself.
has two hydrogen atoms and one oxygen atom. Since oxygen is more electronegative than hydrogen so, it will attract the electrons from hydrogen atom towards itself.
As a result, a partial positive charge will develop on hydrogen atom and a partial negative charge will develop on oxygen atom. Therefore, a molecule of water will become polar in nature.
Being polar in nature, water is able to dissolve many polar molecules. Thus, acts as a universal solvent.
Whereas water is able to absorb heat and changes into vapor state. Whereas it is also able to release energy and thus changes into ice.
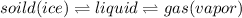
Hence, we can conclude that all of the above are properties of water.